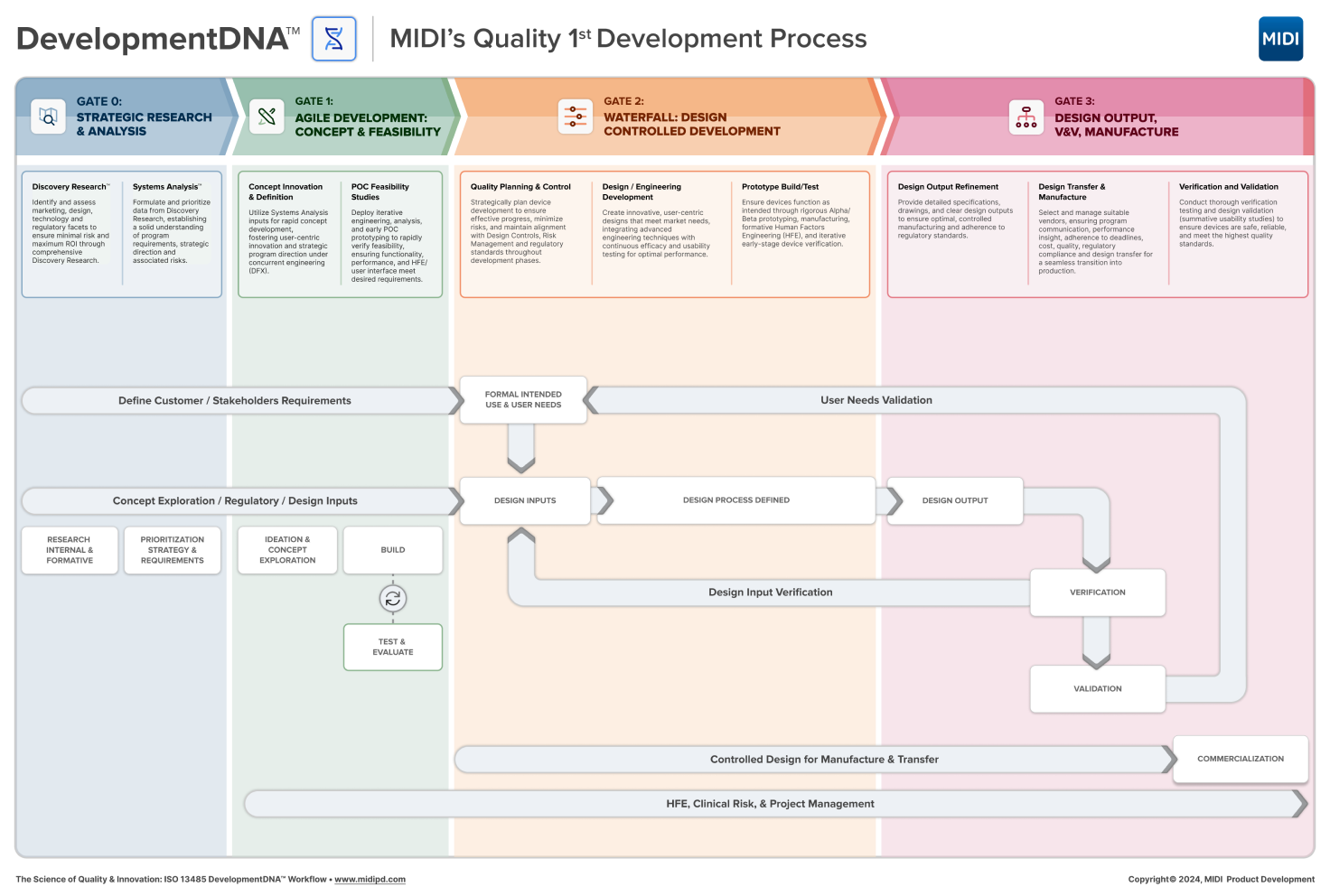
Our Approach
DevelopmentDNA™: MIDI’s strategic process for delivering comprehensive medical device innovation from ideation to commercialization.


Gate 0
Discovery Research™:
MIDI's multidisciplinary teams meet with the client to identify and assess all relevant facets of
marketing, design, usability, technology, intellectual property, engineering, regulatory and
commercialization. Through Discovery Research, we gain comprehensive understanding of all program parameters to ensure minimal initial risk with maximum ROI. Discovery Research sets the stage for strategic program direction and establishes the beginning of requirements capture which will lead to foundational goals for Systems Analysis.
Systems Analysis™:
In this foundational step, MIDI focuses on the collected Discovery Research information, and provides a fast and certain means of formulating and prioritizing requirements data. The Systems Analysis™refinement process yields a solid program understanding, strategic direction, opportunity and associated risks establishing foundational requirements to inform future Design Controls.
Discovery Research™:
MIDI's multidisciplinary teams meet with the client to identify and assess all relevant facets of
marketing, design, usability, technology, intellectual property, engineering, regulatory and
commercialization. Through Discovery Research, we gain comprehensive understanding of all program parameters to ensure minimal initial risk with maximum ROI. Discovery Research sets the stage for strategic program direction and establishes the beginning of requirements capture which will lead to foundational goals for Systems Analysis.
Systems Analysis™:
In this foundational step, MIDI focuses on the collected Discovery Research information, and provides a fast and certain means of formulating and prioritizing requirements data. The Systems Analysis™refinement process yields a solid program understanding, strategic direction, opportunity and associated risks establishing foundational requirements to inform future Design Controls.
Gate 1
Concept Innovation & Definition:
MIDI initiates advanced immersion and planning methods guided by our Systems Analysis inputs to kick start our clients’ projects, ensuring fast start-up and breakthrough innovations while supporting MIDI’s defined regulatory and quality requirements roadmap. Our guided rapid concept development process fosters collective awareness, enabling MIDI to identify strategic program concept paths based on intelligent and informed principles. Our Concurrent Engineering (DFX) and design methods generate product strategy and direction with unprecedented thoroughness, opening new development opportunities for our clients.
POC Feasibility Studies:
MIDI reduces program risk by deploying early and iterative engineering, analysis, design and early Proof of Concept (POC) prototyping research activities to verify feasibility. We rapidly evaluate and validate the feasibility of function, form, performance, and HFE/user interface within compressed timelines. This early agile process step allows us to prove concept, technology, usability and functional requirements for a robust client Design Review.
Concept Innovation & Definition:
MIDI initiates advanced immersion and planning methods guided by our Systems Analysis inputs to kick start our clients’ projects, ensuring fast start-up and breakthrough innovations while supporting MIDI’s defined regulatory and quality requirements roadmap. Our guided rapid concept development process fosters collective awareness, enabling MIDI to identify strategic program concept paths based on intelligent and informed principles. Our Concurrent Engineering (DFX) and design methods generate product strategy and direction with unprecedented thoroughness, opening new development opportunities for our clients.
POC Feasibility Studies:
MIDI reduces program risk by deploying early and iterative engineering, analysis, design and early Proof of Concept (POC) prototyping research activities to verify feasibility. We rapidly evaluate and validate the feasibility of function, form, performance, and HFE/user interface within compressed timelines. This early agile process step allows us to prove concept, technology, usability and functional requirements for a robust client Design Review.
Gate 2
Quality Planning & Control:
Strategically plan device development to ensure highly effective progress while minimizing project risks and ensuring efficient regulatory compliance. Our approach emphasizes thorough regulatory analysis and adherence to compliance standards, facilitating smoother approval processes. We enhance innovation and maintain alignment with Design Controls, Risk Management and regulatory requirements throughout the ongoing development phases.
Design / Engineering Development:
Our multi-disciplinary approach creates innovative, well-rounded direction for design, engineering, and commercialization, crafting a user-centric vision that meets the needs of the market, end-users, and our clients. By integrating advanced engineering techniques and continuous design and usability testing, MIDI generates devices that meet the highest standards of performance, reliability, efficacy, and ROI. Our comprehensive strategy ensures that our products are both innovative and dependable, aligning perfectly with client expectations.
Prototype Build/Test:
Ensure devices function as intended and align with design goals through Alpha/Beta prototyping, manufacturing, formative Human Factors Engineering (HFE), and early-stage device verification. Our rigorous prototype build process identifies potential design flaws and allows for timely adjustments, ensuring optimal performance. By incorporating user feedback and iterative testing, we refine the device to meet both regulatory standards and user needs. This thorough approach guarantees a reliable and user-friendly final product, ready for market entry.
Quality Planning & Control:
Strategically plan device development to ensure highly effective progress while minimizing project risks and ensuring efficient regulatory compliance. Our approach emphasizes thorough regulatory analysis and adherence to compliance standards, facilitating smoother approval processes. We enhance innovation and maintain alignment with Design Controls, Risk Management and regulatory requirements throughout the ongoing development phases.
Design / Engineering Development:
Our multi-disciplinary approach creates innovative, well-rounded direction for design, engineering, and commercialization, crafting a user-centric vision that meets the needs of the market, end-users, and our clients. By integrating advanced engineering techniques and continuous design and usability testing, MIDI generates devices that meet the highest standards of performance, reliability, efficacy, and ROI. Our comprehensive strategy ensures that our products are both innovative and dependable, aligning perfectly with client expectations.
Prototype Build/Test:
Ensure devices function as intended and align with design goals through Alpha/Beta prototyping, manufacturing, formative Human Factors Engineering (HFE), and early-stage device verification. Our rigorous prototype build process identifies potential design flaws and allows for timely adjustments, ensuring optimal performance. By incorporating user feedback and iterative testing, we refine the device to meet both regulatory standards and user needs. This thorough approach guarantees a reliable and user-friendly final product, ready for market entry.
Gate 3
Design Output Refinement:
Provide fully detailed specifications, drawings, and clear design outputs to ensure optimal, controlled manufacturing and regulatory adherence. Our comprehensive refinement process ensures every aspect of the device meets stringent quality standards, facilitating seamless transitions from prototype to production. This robust approach guarantees a high-quality final product that is fully compliant with all relevant regulations.
Design Transfer & Liaison:
Manage supplier sourcing and vendor liaison to ensure seamless integration into the production process. Facilitate the transfer of the design history file and production documentation with reliable support throughout the industrialization process and device launch. Our comprehensive approach ensures a smooth transition from design to manufacturing, minimizing risks and delays. This support guarantees that your device is efficiently prepared for market entry with full compliance and quality assurance.
Verification and Validation:
Conduct thorough verification testing and design validation (summative usability studies) to ensure devices are safe and manufactured to the highest quality. Our rigorous processes identify and rectify potential issues early, supporting product reliability and safety. Our meticulous approach ensures that all devices meet stringent quality standards and regulatory requirements.
Design Output Refinement:
Provide fully detailed specifications, drawings, and clear design outputs to ensure optimal, controlled manufacturing and regulatory adherence. Our comprehensive refinement process ensures every aspect of the device meets stringent quality standards, facilitating seamless transitions from prototype to production. This robust approach guarantees a high-quality final product that is fully compliant with all relevant regulations.
Design Transfer & Liaison:
Manage supplier sourcing and vendor liaison to ensure seamless integration into the production process. Facilitate the transfer of the design history file and production documentation with reliable support throughout the industrialization process and device launch. Our comprehensive approach ensures a smooth transition from design to manufacturing, minimizing risks and delays. This support guarantees that your device is efficiently prepared for market entry with full compliance and quality assurance.
Verification and Validation:
Conduct thorough verification testing and design validation (summative usability studies) to ensure devices are safe and manufactured to the highest quality. Our rigorous processes identify and rectify potential issues early, supporting product reliability and safety. Our meticulous approach ensures that all devices meet stringent quality standards and regulatory requirements.