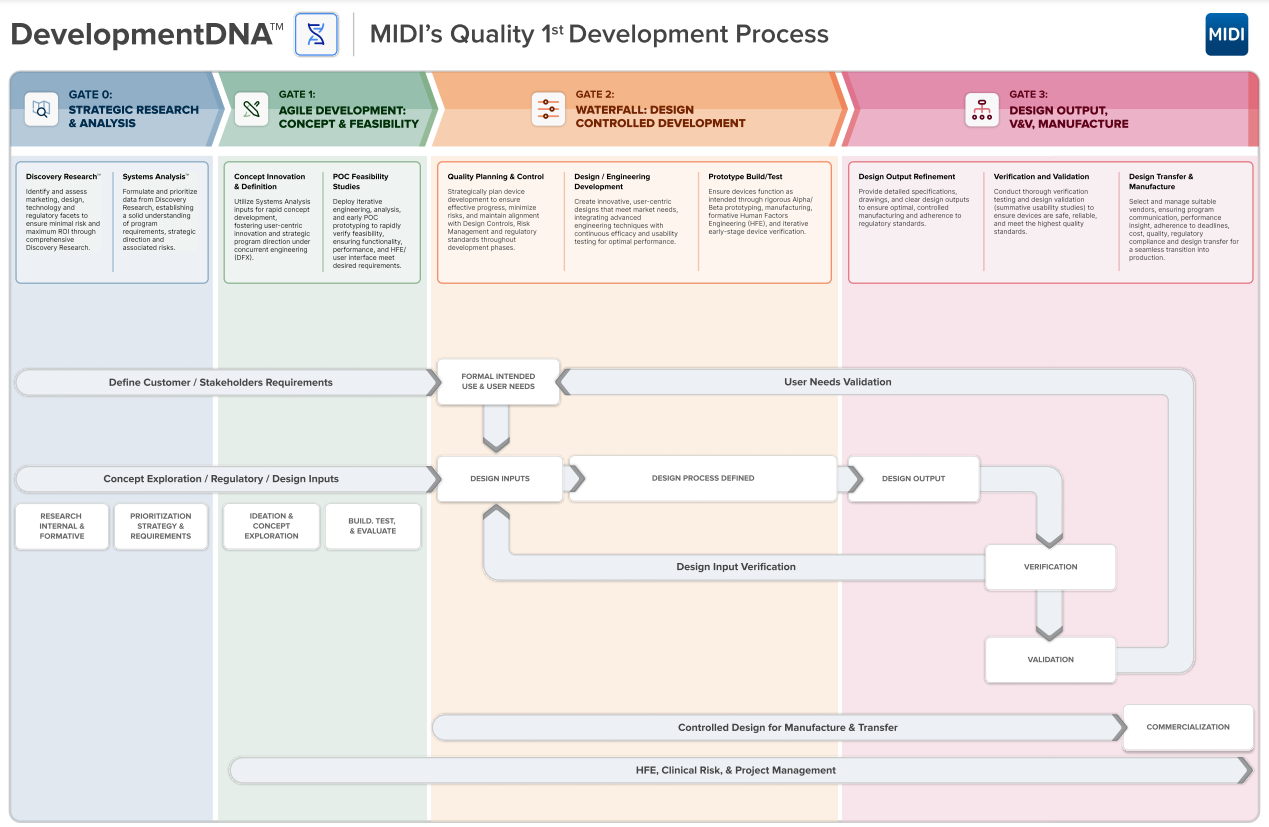
At MIDI, our people-centered, scientific approach underpins the success of every project. We meet the challenges of the competitive MedTech and Biotech marketplace through our proprietary design process, DevelopmentDNA™. This method meticulously covers all development stages, from innovation to regulatory controls and commercialization, ensuring comprehensive outcomes that meet industry requirements.
Our DevelopmentDNA™ approach provides detailed insights into stakeholder identities, behaviors, and needs, combining innovative design, reliable engineering, regulatory controls (ISO 13485, FDA QSR, FDA 510(k), DeNovo and PMA), manufacturing solutions, and product branding. This enables us to deliver targeted solutions based on top-level strategies.
Our DevelopmentDNA™ process includes:
We invite you to download our exclusive guide to learn more about how MIDI’s unique DevelopmentDNA™ approach delivers rapid, user-centric, and collaborative solutions, ensuring your project’s success from ideation to commercialization.
We do not share your information with third parties