
Here at MIDI we’re forging on in the world of medical innovation and product development. As we are faced with months on end of global pandemic, the world needs innovation now more than ever. In response, we’re buckling down, embracing our lasting partnerships and creating in our areas of expertise to do our part in this battle.
We welcome you to follow along with updates of our recent work, shared knowledge and findings, and looking toward the future of innovation. Please join the conversation and share your perspective on the topics at hand by emailing us feedback or connecting on social, we greatly appreciate your input.

Are you listening to the INNOVATION VAULT™ podcast?
In our current podcast series, Paradigm Perception Shift in Medical Device Development: the INNOVATION ROADMAP™, we open up the map and share a detailed look into each roadmap stop sharing our experience and expertise to help guide you in yielding innovation. Listen to the latest episodes
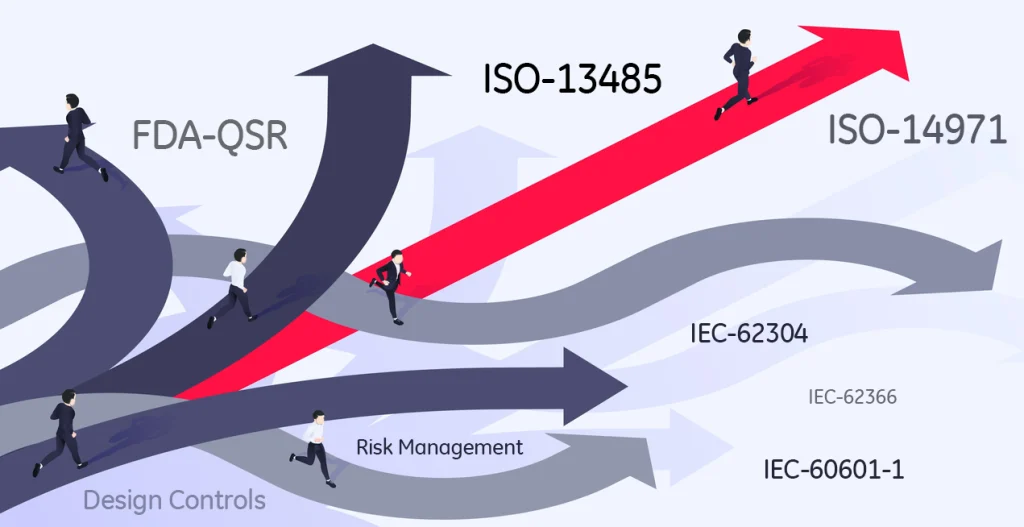
Embracing regulatory controls can help you map medtech innovation
The September issue of Medical Design & Outsourcing (MDO) features Chris Montalbano in a review of MIDI’s INNVOVATION ROADMAP™. Learn more about how using a properly formatted “roadmap” can help a medical device development team realize that the regulations (FDAQSR & ISO-13485) are structured to yield innovation rather than stunt it. Read the full article

MIDI is joining AdvaMed
AdvaMed is a trade association that leads the effort to advance medical technology in order to achieve healthier lives and healthier economies around the world. AdvaMed’s membership has reached over 400 members with a global presence. Member companies include thought leaders, medical technology innovators, medical majors and will now include MIDI. The Association acts as the common voice for companies producing medical devices, diagnostic products and digital health technologies. We’re looking forward to contributing to the AdvaMed community. Learn more about AdvaMed
We do not share your information with third parties